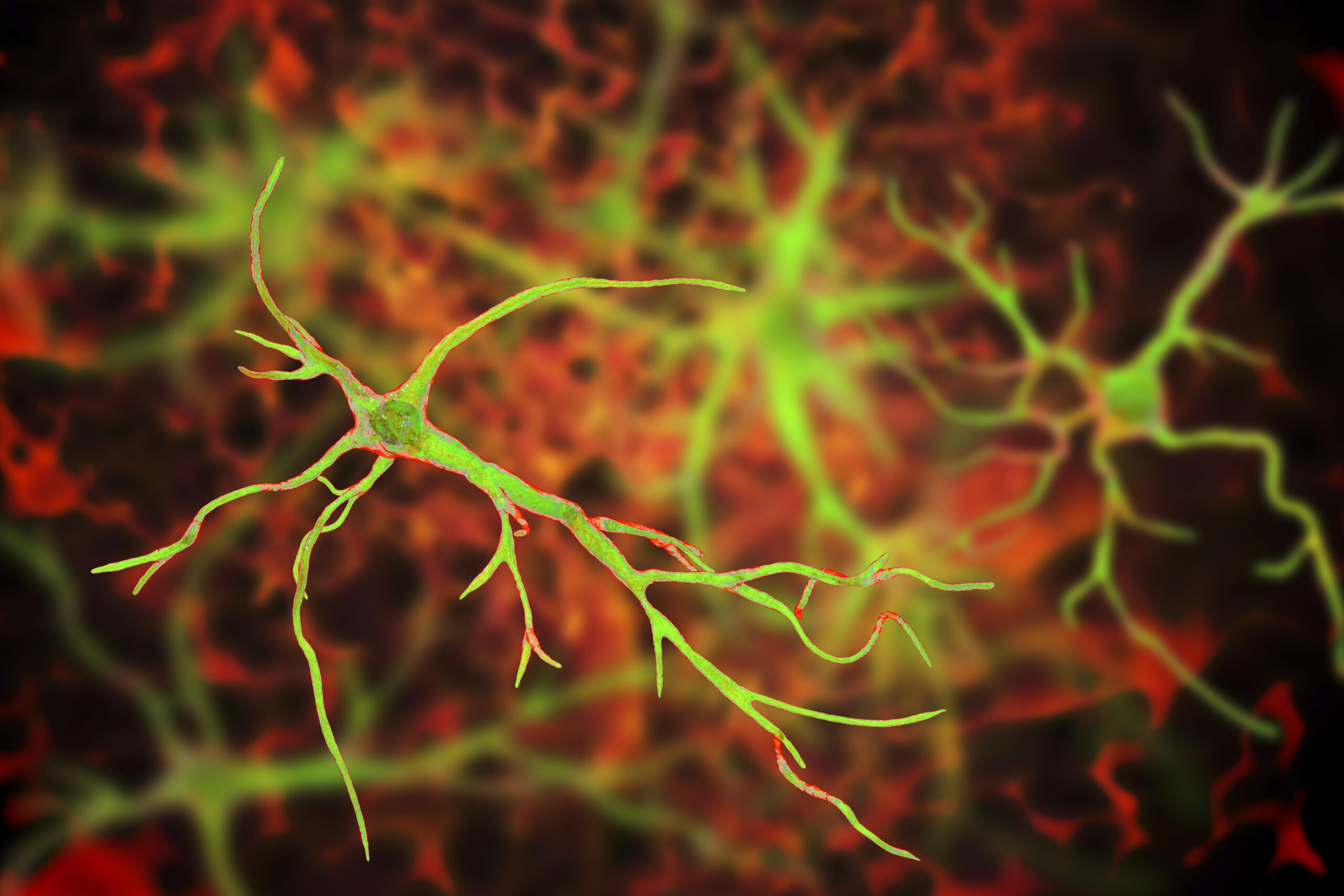
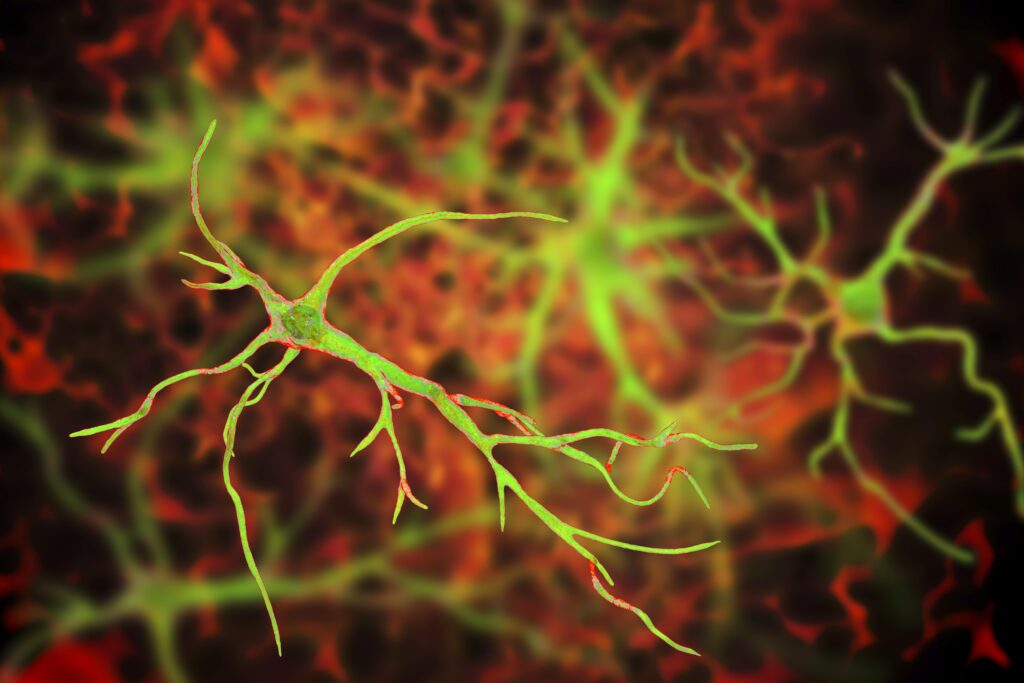
A team of South Korean scientists at the Institute for Basic Science have made a groundbreaking discovery about the role that astrocytes may play in Alzheimer’s disease (AD). The team of researchers, led by Director C. Justin Lee, uncovered a mechanism by which astrocytes in the brain absorb high levels of acetates, which in turn transform them into hazardous reactive astrocytes. Lee and his team created a novel imaging technique that allows for direct observation of astrocyte-neuron interactions, which they hope will lead to more options in the early diagnosis of AD. Overall, the team’s research demonstrates the importance of reactive astrocytes in neuroinflammation and AD pathogenesis and could signal a breakthrough in both the diagnosis and treatment of AD.
Pathogenesis of AD: A Traditional View
Historically, the conventional belief among neuroscientists has been that the accumulation of amyloid beta (Aβ) plaques in the brain is the primary cause of Alzheimer’s and has therefore been the primary target in AD diagnosis and treatment research. While, thus far, treatments that target Aβ plaques have had limited success in treating, or even slowing, the progression of the disease, most AD treatment research has remained focused on Aβ plaques.
Dir. Lee has been researching a different theory: proposing that reactive astrocytes — not Aβ plaques — are the primary cause of AD. And with his team’s new study, he has uncovered a strong body of evidence that supports this theory.
Astrocytes and Alzheimer’s
Along with his team at the Institute for Basic Science’s Center for Cognition and Sociality, Lee had previously been able to demonstrate that reactive astrocytes and the monoamine oxidase B (MAO-B) enzymes were a potentially effective target in AD treatment therapies. And even more recently, Lee discovered that the urea cycle is used by astrocytes and that, when activated, this urea cycle promotes dementia. But researchers had previously been unable to observe or diagnose AD based on astrocyte activity. Lee and his team’s new research changes that.
The new study aimed to identify a clinically validated neuroimaging probe that could visualize reactive astrogliosis, an overactive inflammatory response seen in AD. Lee and his team accomplished this by utilizing positron emission tomography (PET) imaging with C-acetate and F-fluorodeoxyglucose (F-FDG) in order to functionally visualize the neuronal metabolism changes that occur in brains with neuroinflammation and AD.
In doing so, Lee and his team learned that acetate is responsible for promoting reactive astrogliosis, observing that reactive astrocytes take in elevated levels of acetate through elevated monocarboxylate transporter-1 (MCT1) in animal models of both reactive astrogliosis and AD. Not only was elevated acetate uptake linked to reactive astrogliosis, but it also was observed to induce aberrant astrocytic GABA synthesis in the presence of amyloid-beta (Aβ).
Using their novel PET imaging strategy, the team consistently observed alterations in acetate and glucose metabolism in both animal models of AD, such as 5xFAD mice, and human AD patients and were able to demonstrate that a “strong correlation exists between the patient’s cognitive function and the PET signals of both C-acetate and F-FDG.” In other words, the team’s research revealed that acetate is not merely an astrocyte-specific energy source, as previously understood, but that it can also trigger reactive astrogliosis.
Targets for Treatment
Previous research efforts using Aβ as the target for diagnosis and treatment of AD have had extremely limited success. Lee’s team’s study offers a promising alternative pathway for diagnosing and treating the disease. Their study suggests that using C-acetate and F-FDG PET imaging may be a reliable way to achieve early diagnosis of AD. Also, equally important, their research uncovered a potential new treatment target.
As Dir. Lee summarized, “We confirmed a significant recovery when inhibiting MCT1, astrocyte-specific acetate transport, in an AD animal model. We expect MCT1 can be a new therapeutic target for AD.”
Scantox is a part of Scantox, a GLP/GCP-compliant contract research organization (CRO) delivering the highest grade of Discovery, Regulatory Toxicology and CMC/Analytical services since 1977. Scantox focuses on preclinical studies related to central nervous system (CNS) diseases, rare diseases, and mental disorders. With highly predictive disease models available on site and unparalleled preclinical experience, Scantox can handle most CNS drug development needs for biopharmaceutical companies of all sizes. For more information about Scantox, visit www.scantox.com.